Trethera To Participate in Panel Discussion and Research Poster Competition At Los Angeles Bioscience Ecosystem Summit 2025
/EIN News/ -- LOS ANGELES, April 22, 2025 (GLOBE NEWSWIRE) -- Trethera Corporation (“Trethera”), a clinical stage biopharmaceutical company developing first-in-class therapies for cancer and autoimmune diseases, announces a forthcoming panel discussion and poster presentation at the 7th Annual Los Angeles Bioscience Ecosystem Summit 2025 (“LABEST”).
Panel Discussion Details:
- Title: BioSuccess in Los Angeles
- CEO Panelists: Ken Schultz (Trethera), Daniel Gil (Pelage), Dina Leeds (CleopatraRx), and Bethany Mancilla (Capsida)
- Moderator: Amir Naiberg, Associate Vice Chancellor, UCLA; CEO and President, UCLA Technology Development Group
- Location: Centennial Ballroom, 1st floor, Luskin Conference Center
-
Date: Thursday, May 22, 2025 at 2PM
Poster Details:
- Title: First-in-Class Clinical Stage Deoxycytidine Kinase Inhibitor Blocks Inflammatory Bowel Disease Symptoms in an Adoptive CD4 T Cell Transfer Mouse Model
- Authors: Peter M. Clark, PhD; Kenneth A. Schultz, MD; KM Ryan, BS
- Category: Immunity, Inflammation, Infection and Transplantation (I3T)
- Location: Pearl Cohen Scientific Poster Competition, Luskin Conference Center
-
Date: Thursday, May 22, 2025 at 4PM
LABEST 2025 is the premier showcase for bioscience innovation in Los Angeles County and is produced by the UCLA Technology Development Group. The conference attracts over 1,500 life science industry professionals from the world’s leading pharmaceutical companies, venture capital firms, and research institutions. LABEST is intended to promote awareness of the growing life science entrepreneurial ecosystem in Los Angeles and to foster partnerships with the biotechnology and life science industry.
Poster co-author Peter M. Clark is an Associate Professor of Molecular and Medical Pharmacology at the David Geffen School of Medicine and Crump Institute for Molecular Imaging at UCLA. Dr. Clark identified the metabolic enzyme deoxycytidine kinase, dCK, as a key and targetable enzyme required for aberrant T and B cell activation in models of autoimmune diseases, such as Crohn’s disease and lupus. The Clark lab aims to develop novel, first-in-class, drug therapies for cancer and autoimmune diseases.

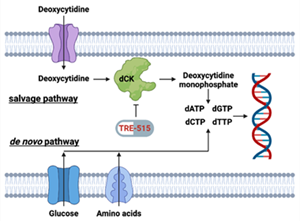
Figure 1: Biochemical pathways for the supply of deoxyribonucleoside triphosphate pools. The salvage pathway becomes upregulated during autoimmune diseases. FDA approved drugs blocking the de novo pathway include Aubagio (teriflunomide), Mavenclad (cladribine), and CellCept (mycophenolate mofetil).
About Trethera
Trethera is a clinical stage, privately held, biopharmaceutical company dedicated to pioneering the development of novel treatments for autoimmune diseases and cancers. Founded by prominent UCLA scientists, Trethera is led by experienced management and board members. Trethera's innovative approach to targeting nucleotide metabolism led to the development of TRE-515, an orally administered capsule twice designated by the FDA as an Orphan Drug. TRE-515 is a first-in-class clinical stage drug that inhibits deoxycytidine kinase (dCK), the rate-limiting enzyme in the nucleoside salvage pathway, one of two biosynthetic pathways that generate DNA precursors. It is believed that some forms of cancer may be preferentially dependent on the salvage pathway to support tumor growth, and certain autoimmune diseases might also respond to TRE-515 treatment. Trethera is developing TRE-515 for use as a monotherapy or in combination to precisely target a metabolic vulnerability of cancer or autoimmune diseases that will transform outcomes for patients.
For more information, please visit us at trethera.com or e-mail Investor Relations at ir@trethera.com. You can also follow Trethera on Facebook and LinkedIn.
Note on Forward-Looking Statements
All statements other than statements of historical facts included in this press release that address activities, events or developments that Trethera believes or anticipates will or may occur in the future are “forward-looking statements,” which may often, but not always, be identified by the use of such words as "may," "might," "will," "will likely result," "would," "should," "estimate," "plan," "project," "forecast," "intend," "expect," "anticipate," "believe," "seek," "continue," "target" or the negative of such terms or other similar expressions. Although Trethera has a reasonable basis for the forward-looking statements contained herein, Trethera cautions that such statements are based on current expectations about future events and are subject to risks, uncertainties and factors relating to medical and scientific research, all of which are difficult to predict and many of which are beyond Trethera’s control, that may cause actual results to differ materially from those expressed or implied by the forward-looking statements in this press release. These potential risks and uncertainties include, without limitation: the extent to which development of any novel cancer therapies or therapies for autoimmune diseases succeeds; whether Trethera would obtain the necessary regulatory approvals to commence human trials or commercialize TRE-515 or any novel therapies resulting from such research; Trethera successfully implementing its growth strategy, including that relating to its disease therapies; the effects of the global Covid-19 pandemic; changes in economic conditions; competition; and risks and uncertainties applicable to the business of Trethera. The statements in this press release speak only as of the date hereof and Trethera does not undertake any obligation to update, amend or clarify these forward-looking statements whether as a result of new information, future events or otherwise. The Company intends that all forward-looking statements be subject to the safe-harbor provisions of the Private Securities Litigation Reform Act of 1995.
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/a3669834-fb66-4439-930d-931d3a67f1b7

Distribution channels: Culture, Society & Lifestyle, Healthcare & Pharmaceuticals Industry, Technology ...
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release